 News
Articles & Recipes
Articles
Should I Store My Essential Oils In The Fridge?...
News
Articles & Recipes
Articles
Should I Store My Essential Oils In The Fridge?...
Should I Store My Essential Oils In The Fridge? and Other Exciting Questions
Essential Oils are complex mixtures of chemicals. It is important to remember that Essential Oils are chemically quite complex. They exist naturally as a combination of aroma chemicals and the balance of these can vary naturally between batches depending on different environmental growing conditions, when the crop was picked and how the crop was processed stored and packed. On top of that there are different chemotypes for the same botanical species, which, put simply are more permanent divergences from a 'norm' based on long-term differences in environment. This is the situation with Lavender where we see a distinct difference in the chemistry between Australian and Bulgarian crops in spite of their same botanical species and processing methods. In short, the chemistry of essential oils is complex and varied. That said it is still possible to map traits between different oil chemistries and to learn from these traits how best to store, handle and work with what is arguably one of nature's greatest gifts to the world - Essential Oils.
It is important to select the right plants / plant parts at the right time and distil them with care.
Harsh Conditions.
When we talk about the storage of essential oils we often find ourselves referring to the following: Store them away from sunlight and in the dark, keep the lids on, keep them in the fridge. While these pointers weren't just picked out of thin air it isn't necessarily practical or necessary to store all essential oils this way, at least not all of the time so, it might pay to investigate each of these conditions a bit further to work out what's best for each oil.
Chemical Reactions.
Temperature
In the world of chemistry we have a thing called the 'Arrhenius equation' which states that a temperature rise of 10°C approximately doubles chemical reaction rates. That is why you often have to perform chemical reactions at high or elevated temperature - the heat is the energy so the more heat, the more energy is in the system. This does go some way towards supporting the idea that we should store reactive ingredients in the fridge.
BUT
Low temperatures favour the solubility of oxygen in liquids = liquids dissolve more oxygen in the cooler temperatures so if we have oils that are generally quite slow to react and put them into the fridge they might actually end up reacting faster as the temperature is cold enough to push more oxygen into the oil!
So what do we do?
Unless stated otherwise the general rule of thumb in terms of temperature is to store at or just under room temperature and to keep the temperature as stable as possible.
Essential Oil components particularly sensitive to temperature are: Limonene, Alpha Pinene, Terpenes. Terpenoids.
UV Light.
Some aromatic chemicals found within essential oils are particularly sensitive to UV light and will break down quickly when exposed this way. This can quite easily be managed in the long-term storage of the oils by keeping them in dark bottles and not storing Essential Oils on a window ledge or outside. However, in a finished cosmetic product it can be more restrictive.
Essential Oil components particularly sensitive to UV are the Bergapten and other furocumarin rich oils, limonene (terpenes)
Oxygen.
Once an essential oil has been saturated with oxygen, peroxides can build up irrespective of how the oil is then stored meaning that a half bottle of oil kept for 5 months may fair no better in the fridge, in a dark temperate corner of your cupboard or in a low temperature oven. This paper 'Essential oils can contain allergenic hydroperoxides at eliciting levels, regardless of handling and storage' explains this.
Oxygen levels in air are related to the humidity and temperature of that air. High humidity = lower levels of oxygen = better for essential oils that oxidise. Low humidity = high level of oxygen = worse for essential oils. But as temperature and humidity are linked it is important to consider both. In fact, in a typical fridge where the temperature is set at 6°C and the humidity is hovering around optimal levels of 85% you might expect an oxygen level of 20.7% whereas in a 26°C room with around 50% humidity (40-50 is comfortable) you would expect 20.55% Oxygen. If the warmer storage could be a little humid, say 70% the oxygen levels would fall to 20.4% which is a 1.45% drop in available oxygen - enough to make a significant difference for some oils.
Essential oil components particularly sensitive to oxygen are: Terpenes and terpene alcohols (Linalool, linalyl alcohol, alpha pinene).
So What is Going On Inside The Oil?
A range of chemical reactions.
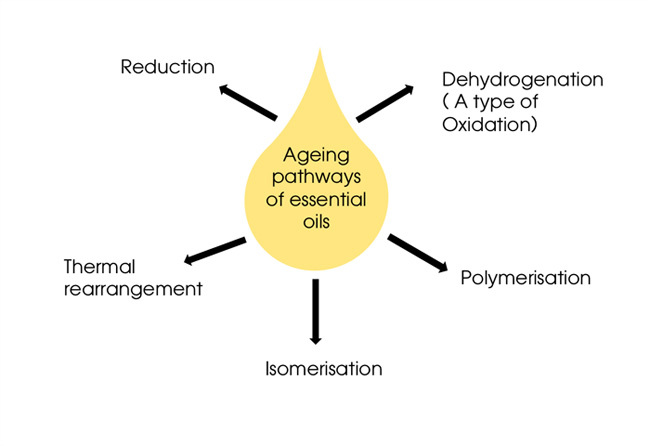
As we have mentioned before, essential oils are complex mixtures of aroma chemicals, some of which are more likely to change than others. In oils that contain a high percentage of highly reactive chemicals it pays to know what triggers their instability so we can work out how best to store and formulate with them. Here are some of the most common reactions that happen within an essential oil.
Isomerisation.
This can happen spontaneously or when a catalyst is present and basically results in a re-arrangement in the atoms of a molecule. This re-arrangement may make the ingredient more or less biologically active. Isomerisation that requires a catalyst to provide the energy can significantly change the polarity and permeability of the aroma chemical. Catalysts may be UV light, metal contaminants or an acidic environment. So one would expect isomerization reactions to speed up as the temperature rises and in the presence of contaminants.
Aroma Chemicals that do this: Carvone, Geraniol, Limonene, Menthol., Thymol, Carcavrol, Neral, Geranial.
Oxidation and Reduction (Dehydrogenation)
Dehydrogenation is a specific type of oxidation where an aroma chemical loses a hydrogen from its structure. This may result in a double bond forming, which is what happens when an alkane reacts to form an alkene. Double bonds tend to be a target for oxidation so this type of reaction generally speeds up when oxygen or another chemical catalyst is present.
The dehydrogenation is often the first in a string of reactions that then go on to leave the essential oil aged. These chemical changes typically affect Alkanes first and then affect aldehydes and ketones, which are commonly found in essential oil.
A storage temperature change from 20°C to 30°C or a dose of UV light would be less significant a trigger to these reactions as the presence of oxygen so storing in air-tight containers or under a nitrogen atmosphere is a wise first step. At the very least decanting oils into small bottles so they are used quickly after opening is a good plan.
Aroma chemicals involved in these reactions include: Monoterpenes such as menthol, Thymol, p-cymene, Carvacrol, Limonene, Geraniol, Citral, Citronellal, Linalool. Terpinen-4-ol, Terpinene, Santalol. PInene. Neral
Thermal rearrangement.
This is another type of isomerization that is purely catalyzed by heat. The hotter the oil, the more likely that this reaction will happen. UV is not involved so light or dark storage would not impact this reaction.
Aroma chemicals involved in these reactions include: Pinene. Limonene, Citral and Neral.
Polymerisation Reaction.
Polymerisation reactions can occur in essential oils and when they do they affect the overall aroma profile, sometimes making the aroma less volatile or weaker. Polymerisation reactions involve two or more molecules reacting together to form a larger molecule. This type of reactionmay be partly to blame for some essential oils becoming more viscous over time (another reason for viscosity increases is dehydration). The reaction always requires a catalyst and that is often (but not always) provided in the form of oxygen. So, we can surmise that polymerization speeds up with increasing oxygen concentration in the oil.
Aroma Chemicals involved in this reaction: Terpenes, lactones, sesquiterpenes.
So how should oils be stored?
Reading all of the above only really serves to highlight how complicated this whole thing can be but we can start to map out some patterns now and using the patterns we find from the scientific research to guide our reading in terms of what stability work has been done on essential oils. What I've found is that there has been quite a bit of work done on herb type essential oils and those used in flavouring but that not all of these research papers are so relevant to cosmetics where stability has to be for 12-30 months and water contents can be quite high. That said from all of the research that has gone into this report I would conclude that the very best fall-back position for essential oil storage is a room temperature, high humidity situation where oxygen levels are limited, possibly even by using a nitrogen or argon purge in opened bottles. While UV irradiation isn't a key destabilizing factor in the majority of oils, oils do still generally benefit (and there is no detriment) to storing in dark containers.
To sum this all up in a couple of words it would be fair to say that oxygen is the biggest problem and avoiding it will give you the best solutions!
| Dominant Chemistry | Examples | Oils With This Chemistry | Biggest Problem | Biggest Trigger | Solution |
| Monoterpenes | Pinene | Myrtle, Kanuka, Juniperberry, Pine, Cypress, Rosemary, Fragonia | Polymerisation, thermal re-arrangement, Oxidation and reduction | Oxygen | Room temp, higher humidity |
| Carene | Blackcurrant, Sage, Pine, Fir, Pepper, Cypress. | ||||
| Cymene | Black seed, Thyme, Camphor | ||||
| Monocyclic Monoterpenoid Alkene Alcohol | Terpene-4-ol | Marjoram, Basil, Juniper, Tea Tree | Oxidation and rediction | Oxygen | Room temp, high humidity |
| Monocyclic Monoterpenoid Alkene | Limoneone | Orange, Grapefruit, Clementine, Tangerine, Lemon, Celery, Mandarin, Tangelo, Dill, Elemi, Lime, Fir needle, Bergamot, Pepper, Camphor, Verbena, Gingergrass, Turpentine | Isomerisation, oxidation and reduction, thermal re-arrangement, | Oxygen | Cooler room temp but high humidity, take care when using in formulations that might contain metalic elements that could act as a catalyst. UV protection essential. |
| Phenols | Thymol | Thyme, Ajowan, Tansy | Oxidation and reduction, Isomerisation | Oxygen | Room temperature, high humidity |
| Phenols | Carvacrol | Marjoram, Thyme | Isomerisation, Oxidation and reduction, | Oxygen | Cooler room temp but high humidity, take care when using in formulations that might contain metalic elements that could act as a catalyst |
| Eugenol | Clove Bud, Cinnamon Leaf, Pimento, Basil | ||||
| Alcohol | Citronellol | Rose, Citronella, Geranium | Oxidation and reduction, Isomerisation | Oxygen | Room temperature, high humidity |
| Farnesol | Sandalwood (10%) | ||||
| Geraniol | Bergamot, Palmarosa, Thyme, Geranium, Citronella, Rose | ||||
| Linalool | Rosewood, Coriander, Thyme, Champaca, Basil, Rosalina, Neroli, Hyssop, Lavender, Ylang Ylang | ||||
| Menthol | Peppermint, Cornmint, Basil (lemon) | ||||
| Ahdehydes | Citral | Myrtle, Lemongrass, Tea Tree, May Chang, Verbena, Honey Myrtle, Melissa, Basil | Thermal rearrangement, Oxidation and reduction | Oxygen | Room temperature to cooler, high humidity |
| Geranial | Lemon Myrtle, Lemongrass, Tea Tree Lemon Scented, May Chang, Melissa, Honey Myrtle | ||||
| Neral | Lemon Myrtle, Lemongrass, May Chang, Tea Tree, Verbena, Honey Myrtle, Melissa, Basil | ||||
| Vanillin | Vanilla | ||||
| Ketones | Camphor | Lavender, Sage, Basil, Rosemary, Rosemary, Yarrow, Tansy | Oxidation and Reduction, Isomerisation | Oxygen/ Light | Room temperature, high humidity |
| Carvone | Caraway, Bill, Verbena, Spearmint | ||||
| Furanocoumarin Ethers | Bergapten | Lime Expressed, Bergamot, Bitter Orange, Grapefruit, Angelica, Tangerine. | Isomerisation | Light | Dark container, room temperature, high humidity |
Finally, some good news. Some Essential Oils Help You and Themselves! While this article has focused on all the very many ways that essential oils can break down and become unstable, it is true to say that in real-life some oils fair better than they theoretically should (on paper). That is often because of their natural antioxidant content.
Eugenol, Vanillin, Thymol, Methofuran, Methyleugenol , Terpinolene, Terpine, Geraniol, Chamazulene, and Benzyl Alcohol all have some anti-oxidant properties and may well help the oil to self-stabilise in the bottle.
On top of that some oils produce anti-peroxide agents such as Cinnamaldehyde found in Cinnamon. This can also help Cinnamon oil last longer than might otherwise have been expected.
Summing up.
The aim of this article was to shine a light on just how complicated essential oil chemistry can be and just how many things can affect an oils stability from the environment it grows in to when it was picked and then into how it is stored. That said, all of this has boiled down to a relatively simple reality that for the most part oils are best stored in full bottles in room temperature and with a higher rather than lower humidity. That wasn't something I was expecting and is actually quite a relief given the costs and space required to set up refrigeration for a haul of oils!
Hopefully some of the more sciency detail stuff will help you to understand why some different oils seem to behave in a similar way and why some seem to go off very quickly in particular products while others last for ages. In general I hope it has provided some food for thought.
What this article wasn't designed to do is offer absolute guarantees of exactly how much extra benefit you might get from storing an oil at one set of conditions over another or for using one oil vs another in a particular formula - That level of detail would require its own stability testing and lab work - but what I do hope is that it off
Amanda Foxon-Hill
10 April 2017
Some Key References:
- Oxidation of aroma chemicals
- Essential oils can contain allergenic hydroperoxides at eliciting levels, regardless of handling and storage’. Contact Dermatitis, Volume 73 (4)- Oct 1 2015.
- Effect of Diurnal Variability and Storage Conditions On Essential Oil Content and Quality of Damask Rose
- Humidity vs Temperature and Oxygen Levels.
- Relative Humidity For Homes.
- Impact of Different Storage Conditions On The Quality of Selected Essential Oil.
